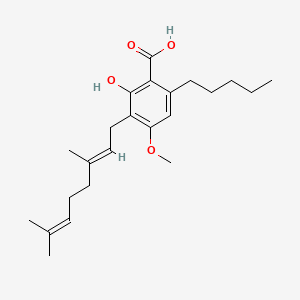
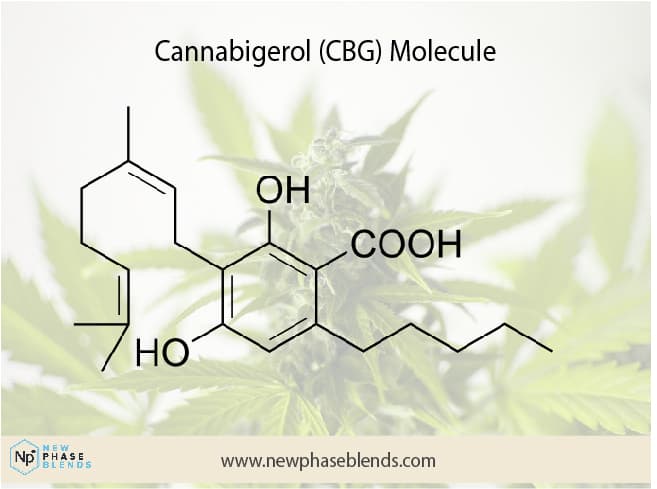
CBG, or cannabigerol, is a class of cannabinoids produced by the cannabis plant. Of this class of major cannabinoids, Cannabigerolic acid monomethyl ether (CBGAM) is a sister molecule.
What is Cannabigerolic Acid Monoethylether (CBGAM)?
There is no accurate record of when CBGAM was discovered. However, we do know when it’s mother cannabinoid, CBG (cannabigerol) was discovered. In 1964, researchers Yehiel Gaoni and Raphael Mechoulam discovered cannabigerol (CBG), a compound isolated from hashish made from the marijuana plant.
During this same experiment, it was also discovered that Cannabigerolic Acid (CBGA) is the primary cannabinoid formed in the cannabis plant. Not only is it the primary cannabinoid, but CBGA is responsible for forming many other cannabinoids found.
There really is not much verifiable information or studies available about CBGAM. We can gather, though, that cannabinoids like CBDA, THCA, CBCA, and CBGMA are isolated using a simple technique called chromatography. This process is used with a clean, domestic cannabis strain. Cannabinoids are then isolated, accordingly.
There is a restriction in the study of some cannabinoids because of their psychoactive effects. CBGAM is at its initial stage of research and development. Almost every compound present in the cannabis plant is known to have distinctive properties that are useful for the human body.
We hope to see more research on CBGAM that will contribute to a more comprehensive understanding of the compound and its’ potential effects.
How Does Cannabigerolic Acid Monoethylether Work?
There is little to no accurate reporting on how CBGAM works in the body. Since CBGAM is part of the cannabigerol class, there is a chance that CBGAM might have a mechanism of action associated with CBG. Again, there just isn’t much we know about this cannabinoid at this time.
Just as it is with other cannabinoids, CBGs functions by impacting the Endocannabinoid System (ECS). Endocannabinoids are produced naturally by the body, and they are responsible for the regulation of physiological functions and maintenance of internal balance.
Although the method of the mechanism is still unknown, researchers speculate that CBG may have a different way of interacting with cannabinoid receptors.
Possible Therapeutic Benefits of CBGAM
Since Cannabigerolic acid Monomethyl ether (CBGAM) belongs to the category of the CBG, there are chances that CBGAM might have similar therapeutic/medicinal benefits to CBG.
Most of the studies performed on CBG were done on lab animals and not humans. However, there are reasons to believe that this compound might have similar effects when used on human beings.
More About CBG
CBG is a non-psychoactive compound that is reported to be an analgesic compound and anti-inflammatory. It has also been reported to treat glaucoma by reducing painful intraocular eye pressure.
This is done through the work of the endocannabinoid system. You can watch this video for more information on this bodily system:
Interesting Facts About CBGAM
| Property Name | Property Value | Reference |
|---|---|---|
| Molecular Weight | 374.5 | Computed by PubChem 2.1 (PubChem release 2021.05.07) |
| XLogP3-AA | 7.8 | Computed by XLogP3 3.0 (PubChem release 2021.05.07) |
| Hydrogen Bond Donor Count | 2 | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Hydrogen Bond Acceptor Count | 4 | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Rotatable Bond Count | 11 | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Exact Mass | 374.24570956 | Computed by PubChem 2.1 (PubChem release 2021.05.07) |
| Monoisotopic Mass | 374.24570956 | Computed by PubChem 2.1 (PubChem release 2021.05.07) |
| Topological Polar Surface Area | 66.8 Ų | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Heavy Atom Count | 27 | Computed by PubChem |
| Formal Charge | 0 | Computed by PubChem |
| Complexity | 505 | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Isotope Atom Count | 0 | Computed by PubChem |
| Defined Atom Stereocenter Count | 0 | Computed by PubChem |
| Undefined Atom Stereocenter Count | 0 | Computed by PubChem |
| Defined Bond Stereocenter Count | 1 | Computed by PubChem |
| Undefined Bond Stereocenter Count | 0 | Computed by PubChem |
| Covalently-Bonded Unit Count | 1 | Computed by PubChem |
| Compound Is Canonicalized | Yes | Computed by PubChem (release 2021.05.07) |
A research carried out on rat models showed CBG protects against neurodegenerative diseases like Huntington’s diseases.
Another promising use of CBG is in the treatment of Inflammatory Bowel Diseases (IBDs), ulcerative colitis and Crohn’s disease.
Since Cannabigerolic acid Monomethyl ether (CBGAM) belongs to the CBG cannabinoid class, there are chances that CBGAM might have similar therapeutic/medicinal benefits to CBG.
References
Valdeolivas S., et al, Neuroprotective properties of cannabigerol in Huntington’s disease: studies in R6/2 mice and 3-nitropropionate lesioned mice. Neurotherapeutics. 2015 Jan;12(1):185-99. doi: 10.1007/s13311-014-0304-z.
Colasanti, BK., A Comparison of the Ocular and Central Effects of Δ9-Tetrahydrocannabinol and Cannabigerol. Journal of Ocular Pharmacology and TherapeuticsVol. 6, No. 4.#
Borellis, F. et al, Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a Cannabis derived non-psychotropic cannabinoid. Carcinogenesis, Volume 35, Issue 12, December 2014, Pages 2787–2797,
Back to List of Cannabinoids